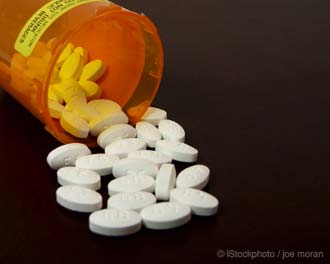
 A September 23, 2010 article in the New England Journal of Medicine announced that, finally, the FDA has stepped forward and decided on regulatory action for Avandia, a diabetes drug that last year claimed 1,354 lives as a result of cardiac-associated problems.
A September 23, 2010 article in the New England Journal of Medicine announced that, finally, the FDA has stepped forward and decided on regulatory action for Avandia, a diabetes drug that last year claimed 1,354 lives as a result of cardiac-associated problems.
Under the ruling, the drug will be available to patients not already taking it only if they are unable to achieve glycemic control using other medications and, in consultation with their health care professional, decide not to take a different drug for medical reasons.
Current users of Avandia will be able to continue using the medication if they appear to be benefiting from it and they acknowledge that they understand these risks. Doctors will have to attest to and document their patients’ eligibility; patients will have to review statements describing the cardiovascular safety concerns.
But did the FDA go far enough – could it be too little, too late?



 The US supreme court on Monday heard arguments in a case that could threaten Americans’ access...
The US supreme court on Monday heard arguments in a case that could threaten Americans’ access... Toothpaste can be widely contaminated with lead and other dangerous heavy metals, new research shows.
Most of...
Toothpaste can be widely contaminated with lead and other dangerous heavy metals, new research shows.
Most of...